Chemical Properties of Phenols
Chemical Properties of Phenols: Overview
This topic covers concepts, such as, Chemical Properties of Phenols, Reaction of Phenols with Active Metals, Preparation of Aspirin & Oxidation of Phenols etc.
Important Questions on Chemical Properties of Phenols
Which of the following statements are true?
(i) Phenol is a stronger acid than alcohol.
(ii) Alcohols are comparatively more soluble in water than the corresponding hydrocarbons.
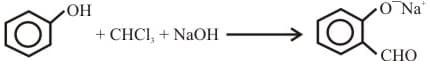
The electrophile involved in the above reaction is
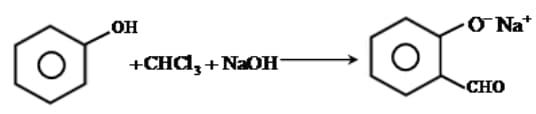
The electrophile involved in the above reaction is
The structure of the compound that gives a tribromo derivative on treatment with bromine water is –
The structure of the compound that gives a tribromo derivative on treatment with bromine water is
p-cresol reacts with chloroform in alkaline medium to give the compound which adds hydrogen cyanide to form the compound . The latter on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is –
The major product obtained on interaction of phenol with sodium hydroxide and carbon dioxide is –
Explain about the acidic character of phenol by using suitable resonating structures. What will be the effect of substituents on acidity?
Phenol on oxidation in the air gives _____.
Oxidation of phenol with chromyl chloride produces a conjugate _____.
What happens when phenol react with chromyl chloride.
Explain the oxidation of phenol in the presence of chromyl chloride.
In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion : Phenols give and nitrophenol on nitration with conc. and mixture.
Reason : group in phenol is directing.
Assertion : Phenol is more reactive than benzene towards electrophilic substitution reaction.
Reason : In the case of phenol, the intermediate arenium ion is more stabilized by resonance.
Assertion: Phenol is more acidic than ethanol.
Reason: Phenoxide ion is resonance stabilized.
Electrophilic substitution involved in _____ of phenol.
At high temperature, phenol produces para product on sulphonation.
What is sulphonation reaction of phenol?
Write the reaction of phenol with sulphuric acid.
Acetoxy benzoic acid is commonly known as
